In the world of manufacturing, pharmaceuticals, food production, and many other industries, ensuring product quality and consistency is paramount. One essential tool for achieving this is the Certificate of Analysis (COA). A COA is a document issued by a qualified entity (usually a laboratory or the manufacturer itself) that confirms that a product meets specific quality standards and specifications. It’s a critical record that provides detailed information about the product’s characteristics, test results, and compliance. Utilizing a well-structured and comprehensive Certificate of Analysis template streamlines the process of generating these vital documents, reduces errors, and ensures consistent reporting across batches and product lines.
But what exactly goes into a good Certificate of Analysis template? What are the key elements that should be included to provide a clear, accurate, and reliable assessment of a product’s quality? How can you design a COA template that’s both user-friendly and informative? Let’s dive into the essential components and best practices for creating effective Certificate of Analysis templates.
Essential Elements of a Certificate of Analysis Template
A robust COA template should include a variety of elements to provide a complete picture of the product’s analysis. These elements can be broadly categorized into product identification, testing information, results, and approval details.
-
Product Information:
- Product Name: Clear, unambiguous name of the product being analyzed.
- Product Code/SKU: Unique identifier for tracking the product.
- Batch/Lot Number: Identifies the specific production batch being analyzed. This is crucial for traceability.
- Manufacturing Date: Date when the product was manufactured.
- Expiration Date (if applicable): Date after which the product is no longer guaranteed to meet specifications.
- Supplier/Manufacturer Information: Name and contact details of the product’s manufacturer or supplier.
-
Testing Information:
- Date of Analysis: Date on which the analysis was performed.
- Testing Laboratory: Name and address of the laboratory that performed the analysis. Accreditation information (e.g., ISO certification) should also be included if applicable.
- Test Methods: Specific methodologies used for each test. This should include references to recognized standards (e.g., USP, EP, ASTM) or internal procedures. Detailed method descriptions are often documented elsewhere and referenced in the COA.
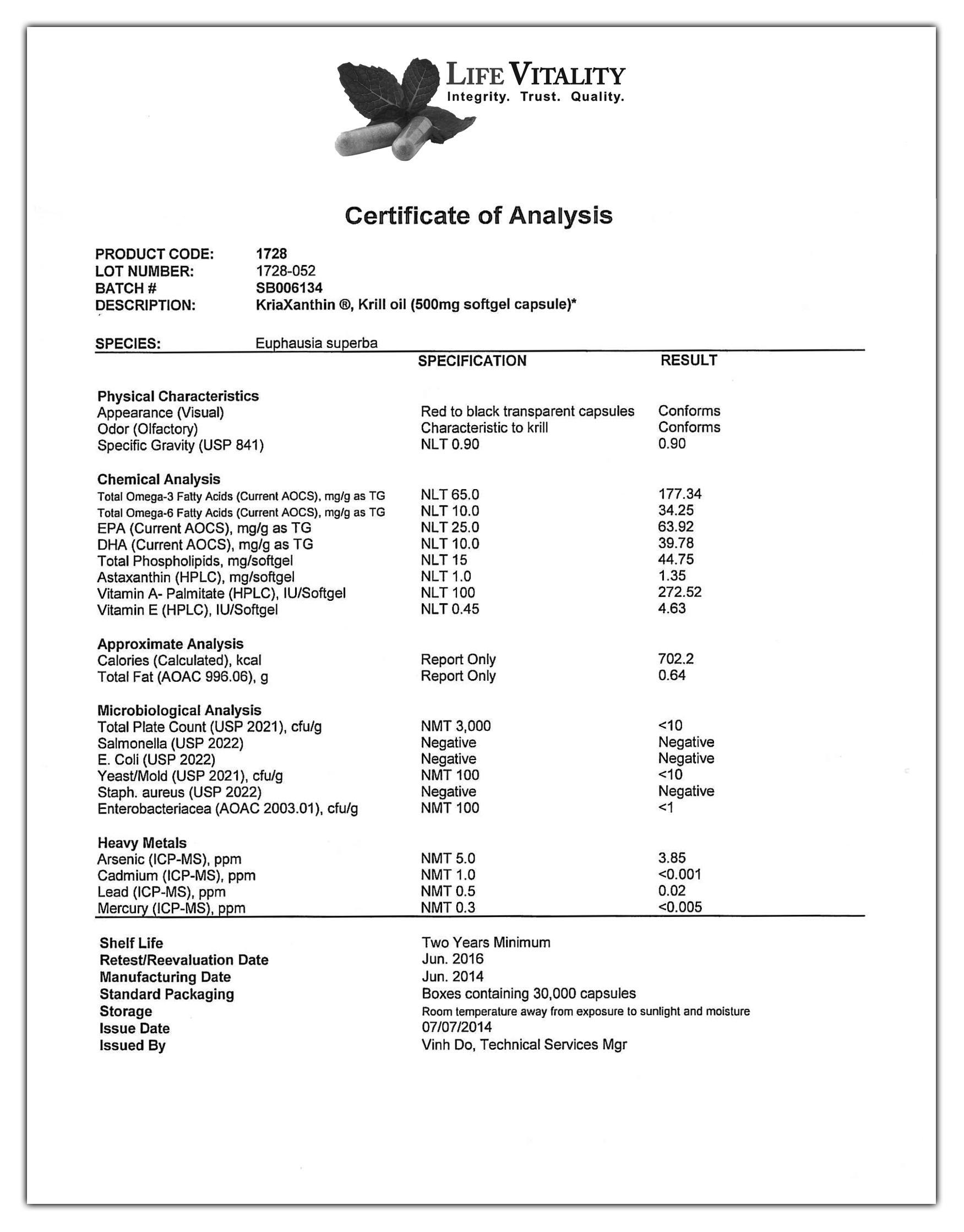
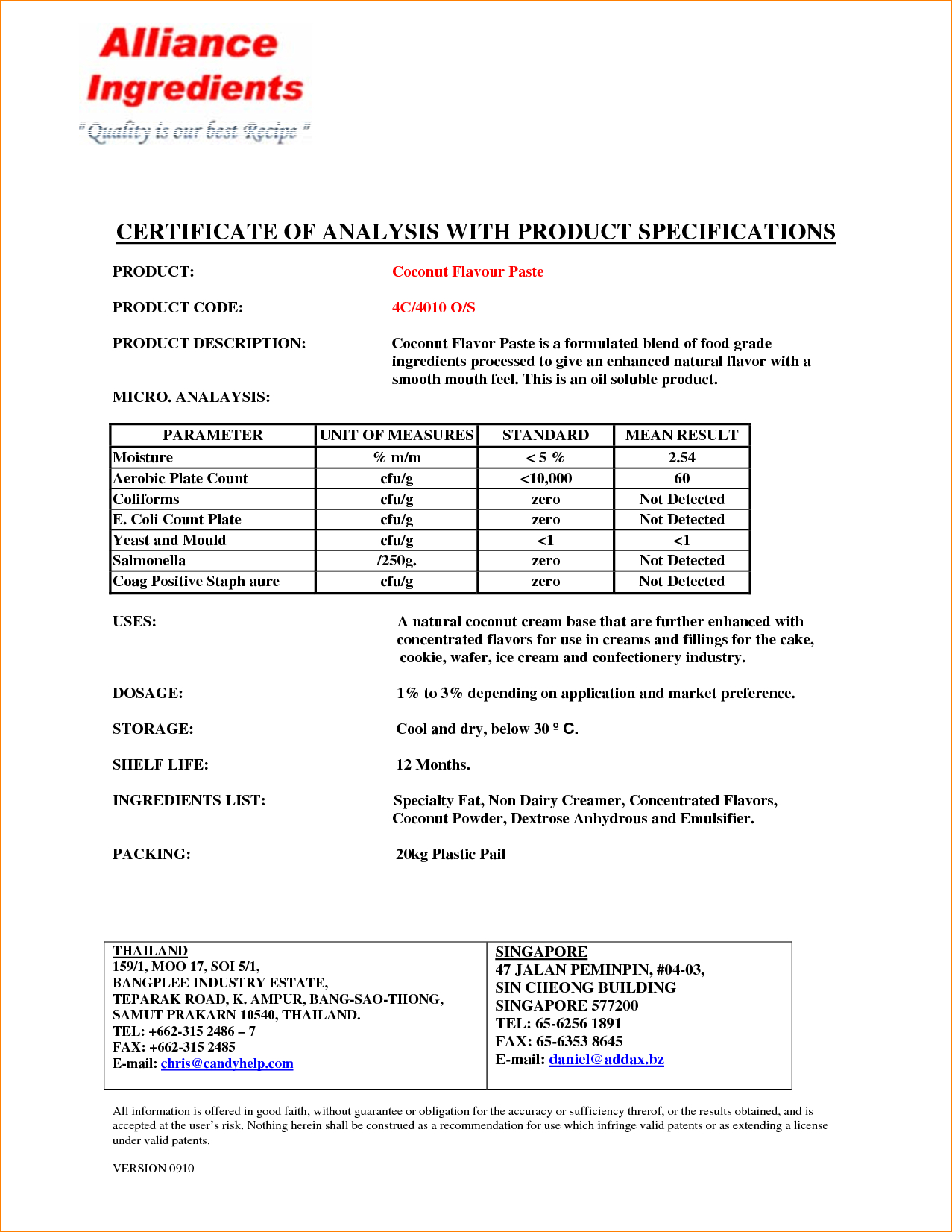
- List of Tests Performed: A comprehensive list of all tests performed on the product. This might include physical properties (e.g., appearance, viscosity), chemical properties (e.g., purity, concentration), microbiological properties (e.g., total plate count, presence of specific pathogens), and other relevant tests.
-
Test Results:
- Test Result for Each Test: The actual result obtained for each test performed. These should be presented clearly and concisely, with appropriate units of measurement.
- Specification Limits: The acceptable range for each test result. This allows for easy comparison of the results against the required standards. Often expressed as a minimum and maximum value.
- Units of Measurement: Clearly defined units for all measurements (e.g., %, ppm, mg/mL).
- Pass/Fail Status: Indication of whether the test result falls within the specified limits.
- Uncertainty of Measurement (Optional): Especially important for critical parameters, this indicates the possible range of values within which the true value likely lies.
-
Approval and Signatory Information:
- Analyst’s Name and Signature: Identifies the person who performed the analysis.
- Reviewer’s Name and Signature: Identifies the person who reviewed and approved the COA.
- Date of Approval: Date on which the COA was approved.
- Company Stamp/Logo: Adds authenticity and branding to the document.
- Disclaimers: Any relevant disclaimers regarding the limitations of the analysis or the use of the product. For example, “This COA applies only to the batch of product identified above.”
Furthermore, your Certificate of Analysis template should be designed for clarity and ease of use. Consider using a table format to present the test results and specifications. Use clear and concise language, avoiding technical jargon where possible. Ensure the font size is readable and the layout is well-organized. Version control is also essential. Each time the template is updated, the version number should be incremented to ensure the latest version is used and previous versions are traceable.
In conclusion, a well-designed Certificate of Analysis template is a cornerstone of quality control. By including the essential elements outlined above and focusing on clarity and accuracy, you can create a COA that effectively communicates product quality and ensures compliance with relevant standards. This not only protects your customers but also strengthens your brand reputation and builds trust in your products. Remember to regularly review and update your template to reflect changes in testing methods, regulations, and product specifications.
If you are looking for Certificate of Analyst Template Blank Printable PDF, Word | Analyst you’ve came to the right page. We have 22 Pics about Certificate of Analyst Template Blank Printable PDF, Word | Analyst like ?4+ Free Sample Certificate of Analysis (COA) Templates?, Certificate of Analysis Template | PDF & Word Download and also Certificate of Analysis Template | PDF & Word Download. Read more:
Certificate Of Analyst Template Blank Printable PDF, Word | Analyst

www.pinterest.com
Certificate Of Origin Form Template – Artofit

www.artofit.org
Simple Certificate Of Analysis Template In Pages, Word, Google Docs

www.template.net
PPT – CERTIFICATE OF ANALYSIS PowerPoint Presentation, Free Download

www.slideserve.com
Certificate Of Analysis Template | PDF & Word Download

templatediy.com
Certificate Of Analysis Blank Printable Template In PDF, Word

templatediy.com
Simple Certificate Of Analysis Template In Pages, Word, Google Docs
www.template.net
Sigma Aldrich Certificate Of Analysis | Certificate Of
www.certificateof.com
Simple Certificate Of Analysis Template In Pages, Word, Google Docs
www.template.net
Certificate Of Analysis COA Fillable PDF Template – Etsy Hong Kong

www.etsy.com
Certified Cbd | Certificate Of Analysis | Cbdintact Inside Certificate

healthylivingforest.com
Certificate Of Analysis Template – Australia

certificate-au.com
5+ Certificate Of Analysis Template | Outline Templates For Certificate
sample.gelorailmu.com
Certificate Of Analysis – Next Valley
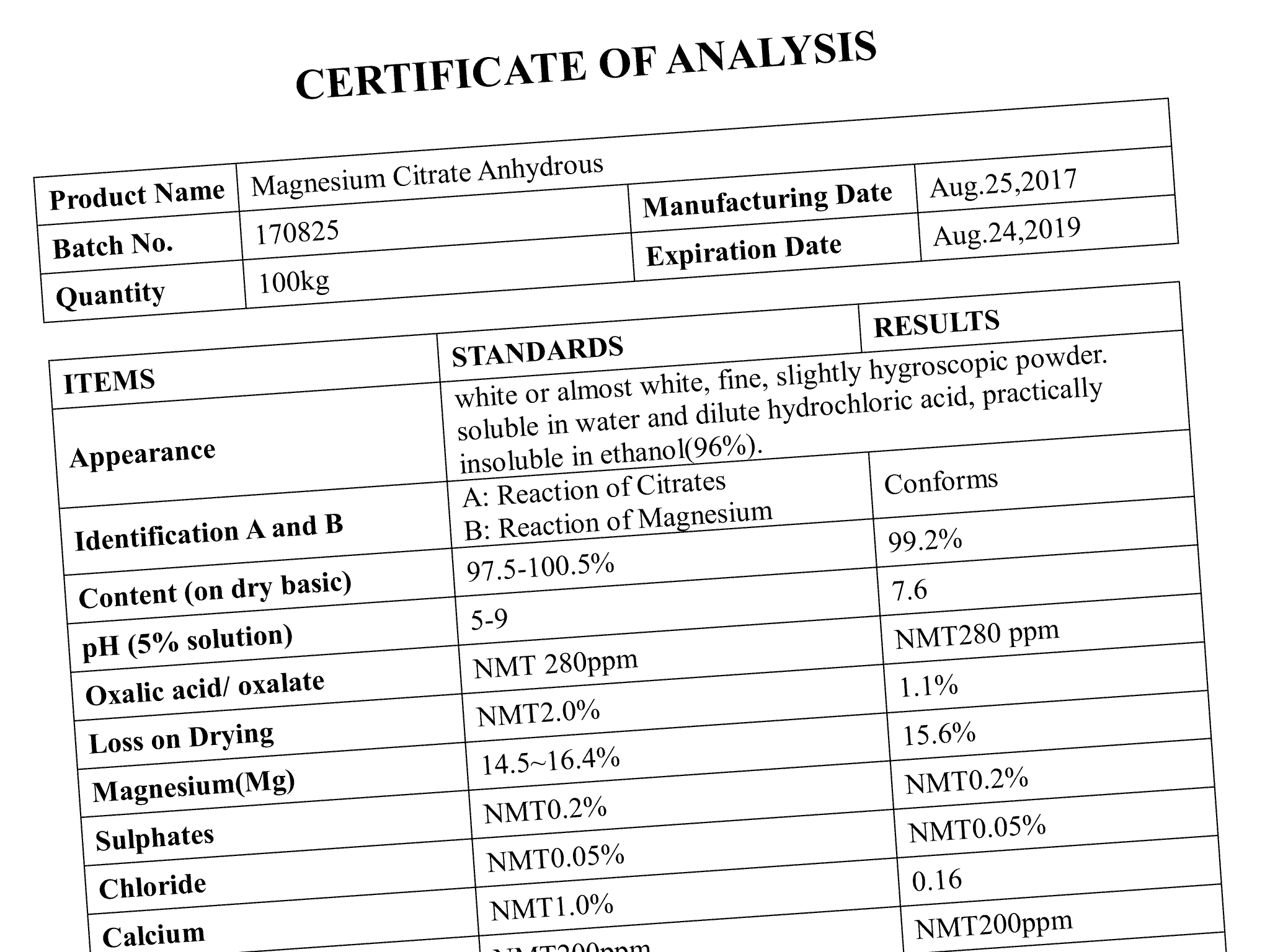
nextvalley.com
Free Certificate Of Analysis Template In PSD, MS Word, Publisher

www.template.net
Free Certificate Of Analysis Templates (Guide & Examples)

www.wordtemplatesonline.net
Free Certificate Of Analysis Templates (Guide & Examples)

www.wordtemplatesonline.net
Certificate Of Analysis Template Lera Mera Within Certificate Of

vancecountyfair.com
?4+ Free Sample Certificate Of Analysis (COA) Templates?

www.certificateof.com
Simple Certificate Of Analysis Template In Pages, Word, Google Docs
www.template.net
Certificate Of Analysis
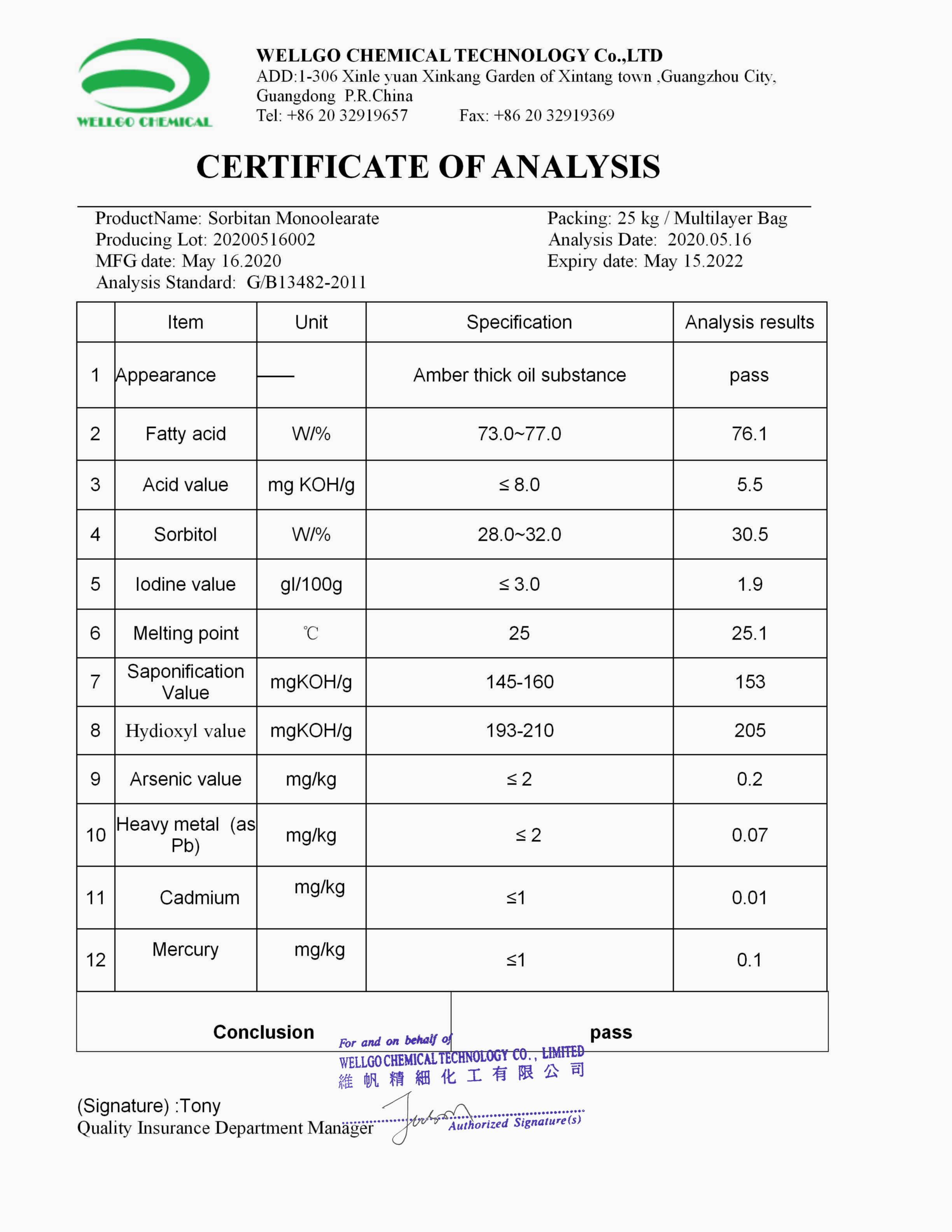
wellgochem.com
Editable Certificate Of Analysis Templates – Edit Online & Download

www.template.net
Free certificate of analysis templates (guide & examples). Certificate of origin form template – artofit. certificate of analysis template lera mera within certificate of …